Campaign for equal access to treatments for SMA adults
SMAtreatmentsforall
Take Action 🖱
Write to your TD | Socials | Videos | Campaign flyer | Endorsements | Thank You | About | Contact
Campaign update
Muscular Dystrophy Ireland (MDI) and Spinal Muscular Atrophy (SMA) would like to thank everyone that has supported our #SMATreatmentsForAll campaign so far. Thanks to your efforts, we have seen significant engagement from your local TDs and senators, as well as Health Department officials and industry professionals. On Tuesday, 27th May at a briefing in Leinster House, SMA Ireland and MDI issued an urgent call for the arbitrary age restriction currently preventing approximately twenty adults in Ireland from getting access to life-saving SMA treatments.
There is still work to be done, and we are asking for further support in keeping the pressure on your representatives until every adult living with SMA has equal access to these life-changing treatments. Please, visit the link and ask your TD for their continued support in overturning this restriction.
Since 2019, there have been three SMA treatments available in Ireland (Spinraza, Zolgensma, and more recently, Risdiplam). The treatments were made available to those living with SMA under the age of 18 at the time of approval. This cohort continue to receive treatment. Meanwhile, those over 18 at the time of approval were denied access and continue to be excluded from treatment.
In her address to TDs, senators, healthcare professionals and industry professionals at the audio-visual room in Leinster House, Dr Aoife McNicholl, 30, Assistant Professor of Psychology at DCU, described her experience as an adult living with SMA. Currently, Dr McNicholl is impacted by the HSE restriction and described it as a “critical barrier” impeding her full and active life.
“I have a boyfriend, a career, and represented my country in wheelchair soccer this weekend. Every day that I go without access to treatment means further deterioration and decline. I know that I will not walk, but I am not looking for a silver bullet. I am looking for a silver lining,” said Dr McNicholl.
The three treatments are available across Europe, the UK and Australia, with ample data to prove the positive impact the treatments have on quality of life and outcomes for adults living with SMA.
Dr McNicholl added: “In Ireland, we are lagging behind other advanced countries. Lifting this restriction would be cost-effective, reducing the risk of future medical interventions and hospital stays.”
Also speaking at the briefing was Professor Orla Hardiman, Professor of Neurology at Trinity College Dublin and Consultant Neurologist at Beaumont Hospital.
“Children born today with SMA can be treated. The current situation is unequitable, unfair and unacceptable, and could be changed with the stroke of a pen.”
In his address at the briefing event, Senator Tom Clonan described the restriction as “casual cruelty” and in “contravention of lawful requirements of the disability act”.
Many TDs and representatives present at the event have raised parliamentary questions, proving that the campaign has so far been successful in raising awareness about this medical inequality. However, we are asking for your continued support as we cannot stop until we have ensured that all adults living with SMA have access to life-changing treatments. Please, use your voice by emailing your local TDs on bit.ly/4mpYWnk , urging their continued action until there are #SMATreatmentsForAll.
Campaign for equal access to treatments for SMA adults
Snapshot of treatment availability in Europe
Availability and reimbursement eligibility for the treatments, nusinersen and risdiplam, among adults living with SMA. Click here for more information.
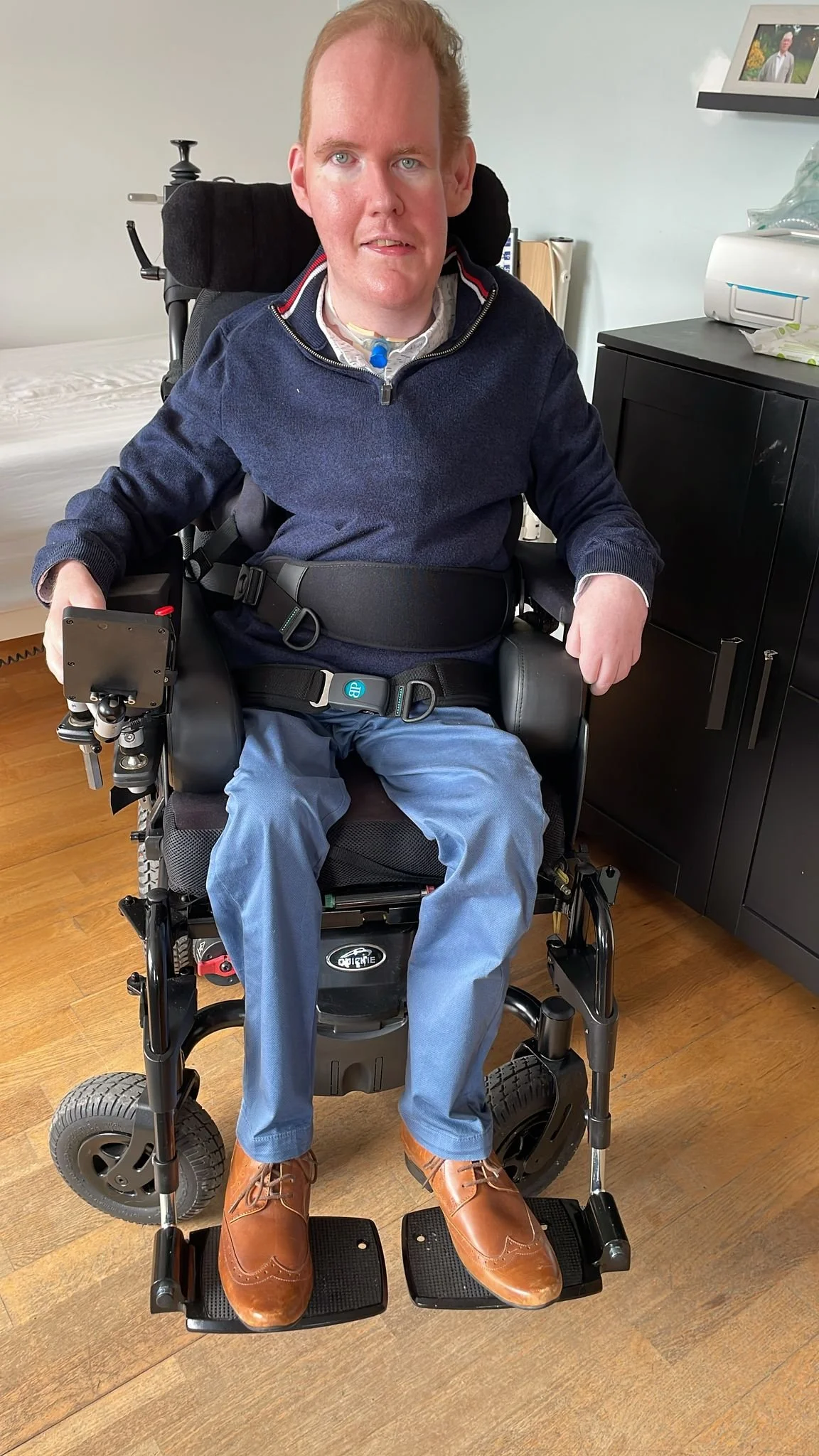
We are campaigning for equal access to life-saving treatments for all adults with spinal muscular atrophy (SMA). Around 20 adults in Ireland are being denied these treatments simply because they were over 18 when the HSE approved them – unlike those who were younger at the time. This age-based healthcare barrier is unjust – and it must end!
Spinal muscular atrophy (SMA) is a rare, progressive neuromuscular condition that causes weakness throughout the body. This is due to the loss of motor neurons – nerve cells responsible for controlling muscles. People with SMA experience profound mobility challenges and often face difficulty swallowing, breathing, and performing basic activities of daily living. In some cases, SMA can be life-limiting, if not properly managed or treated.
Fortunately, in recent years, the HSE has approved three treatments for SMA that can halt the condition's progression and significantly improve life quality for those affected. However, a critical barrier exists: the HSE has imposed an arbitrary age limit of 18 for access to these life-saving treatments.
While individuals under 18 at the time these treatments were approved can continue receiving treatments for life, those over 18 at the time have been excluded from access to any treatments. This creates a deeply unjust and inequitable situation where, for example, a 16-year-old who began treatment four years ago can continue receiving it at the age of 20, while their 19-year-old counterpart, who was also diagnosed and had the same needs, is denied access to the same treatment simply because of an arbitrary age restriction. There are approximately 20 adults in Ireland currently affected by this policy, discriminated against solely due to their age at the time the HSE approved treatments.
Restricting access to life-saving treatments based solely on age is not only unjust; it is cruel and inequitable. Adults with SMA deserve the same opportunity for treatments as those under 18, and we need your support to change this policy.
Ireland's treatment policy for adults with SMA lags behind those of its European counterparts. Countries such as Germany, Italy, the UK, France, Spain, Poland, and Hungary and others have no age restrictions on access to these life-changing treatments. This allows individuals with SMA, regardless of age, to lead improved lives, halt progression and manage their conditions more effectively.
Why are we campaigning?

Take action to support our campaign!
Click here to write to your TD/senator 🖱
Help us spread the word by sharing our posts on social media. Encourage your friends, family, and network to join in!
Watch and share: SMA stories of access and denial
Hear from adults who have access to the life-changing benefits of SMA treatments and from those who can’t!
Emer, is 27 years old and lives in the Republic of Ireland. She describes how she can’t get treatments due to her age, and explains our campaign for equal access to treatments for SMA adults in Ireland.
Aoife is 30 years old and lives in the Republic of Ireland. For every day that goes by without access to treatment, she experiences deterioration and a loss of muscle function..
Naglis is 23 years old and lives in Ireland. Because he was 17 when his treatment was approved, he’s still able to access the treatment now as an adult. He describes improvements to his energy levels and back and neck strength.
Conor is 37 years old and lives in Northern Ireland. He tells us about many huge improvements that he’s experiencing from his treatment, since starting on it 3 years ago.
Tristram is 34 and lives in Australia. He began treatment 2 years ago and says it’s been absolutely amazing, preventing any sort of decline in his health and allowing him to continue living independently.
Thorbjørn is 23 years old and lives in Denmark. Since starting treatment 2 years ago, he can see a huge improvement in his ability to speak more loudly and clearly, as well as finding it a lot easier to eat.
Brad is 26 years old and lives in England. After just 6 doses of his treatment, he’s experiencing a lot of improvements.
Chantel is 34 years old and lives in Australia. She describes the many benefits she’s enjoying since starting her treatment 2.5 years ago.
Natalie lives in the USA and began SMA treatments when she was 25/26 years old. She strongly promotes making treatments available to adults and says that every day without treatment is another day of loss of potential gain from them.
Download our campaign flyer

This campaign has the support of the following organisations: Disability Federation of Ireland, Irish Wheelchair Association, Neurological Alliance of Ireland, Rare Disease Ireland, SMA Europe.
Our campaign is also proud to be endorsed by the following healthcare professionals:
Triona Bannerman, Clinical Nurse Specialist, Beaumont Hospital
Professor Richard Costello, Consultant Respiratory Physician, Beaumont Hospital
Danielle Furlong, Senior Occupational Therapist (Neuromuscular), Children’s Health Ireland at Temple Street
Dr Laura Gallagher, Consultant Clinical Neuropsychologist
Professor Orla Hardiman, Professor of Neurology and Head of the Academic Unit of Neurology at Trinity College Dublin and Consultant Neurologist at Beaumont Hospital
Dr Stela Lefter, Consultant Neurologist, Beaumont Hospital
Professor Denise McDonald, Consultant Paediatrician with a special interest in neurodisability, Children’s Health Ireland at Tallaght
Professor Sinéad Murphy, Consultant Neurologist, Tallaght University Hospital
Meadhbh O’Hanlon, Senior Neuromuscular Physiotherapist, Children’s Health Ireland at Tallaght
Professor Aisling Ryan, Consultant Neurologist, Cork University Hospital
Shane Smyth, Consultant Neurologist, Mater Misericordiae University Hospital
Professor Imran Sulaiman, Consultant Respiratory Physician, Beaumont Hospital
Viora Zachariah, Candidate CNS, Neuromuscular, Cork University Hospital.
Thank You!
Our huge thanks to everyone in the SMA community, far and wide, who supported this campaign; to Aoife, Brad, Chantel, Conor, Emer, Naglis, Natalie, Thorbjørn and Tristram, who shared their experiences on video, to the volunteers on the campaign project team, Aoife, Emer and Jonathan, to Naglis for editing the videos and to Sinéad whose fundraising efforts made it possible for us to carry out this campaign. Sincere thanks also to all the individuals and organisations who have shown their solidarity by expressing their support.
-
Spinal Muscular Atrophy (SMA) is a rare, genetic neuromuscular condition causing progressive muscle wasting (atrophy) and weakness leading to loss of movement. This may affect walking and upper body movement, breathing and swallowing. Importantly, SMA does not affect a person’s ability to think, learn or build relationships with others.
People diagnosed with SMA lack a protein called 'survival motor neuron' (SMN), which is essential for the normal functioning and survival of motor neurons. Without this protein, the motor neurons deteriorate and eventually die. This causes the muscles to fall into disuse, leading to muscle wasting (atrophy) and weakness.
The SMN protein is made by two genes, the SMN1 and SMN2 genes. Most patients with spinal muscular atrophy lack the SMN1 gene but have the SMN2 gene, which mostly produces a 'short' SMN protein which cannot work properly on its own. There are different forms of SMA and a wide spectrum of how severely children and adults are affected. The most common form is known as ‘5q SMA’; referring to the chromosome containing the genetic cause.
When a child or adult is first diagnosed, the doctor gives a clinical classification - SMA Type 0, I, II, III or IV - which reflects the age of onset of symptoms and the motor milestones that someone would be expected to achieve. The severity of the condition varies from person to person, both within and between ‘Types’ - each is affected differently.
The five primary types of SMA
SMA Type I – severe
The most severe and the most common — is usually diagnosed during an infant’s first six months. Babies with SMA Type 1 face many physical challenges, including muscle weakness and trouble breathing, coughing, and swallowing. They may need breathing assistance or a feeding tube. If not treated, type I can be fatal early on in life. 60 per cent of all SMA cases are Type I. SMA Type I is also known as Werdnig-Hoffmann disease.
SMA Type 0 - congenital
SMA Type 0 can be thought of as a subset of SMA Type I, the difference being that symptoms are present at birth or develop very shortly thereafter.
SMA Type II - intermediate
Type II is usually diagnosed after six months of age, but before two years of age. The first sign is often a delay in meeting motor milestones, or failing to meet milestones entirely. Individuals affected by SMA Type II can typically sit up without help, though they may need assistance getting into a seated position, but they are unable to walk and will require a wheelchair.
SMA Type III - milder
Type III SMA is usually diagnosed after 18 months of age, but before three years of age. However, SMA Type III can be diagnosed as late as the teenage years. Individuals affected by SMA Type III are initially able to walk, but have increasingly limited mobility as they grow and eventually, many need to use a wheelchair. Type III is also called Kugelberg-Welander disease or juvenile SMA.
SMA Type IV - adult onset
SMA Type IV is very rare. It usually surfaces in adulthood, and it leads to mild motor impairment. While symptoms can begin as early as age 18, they usually begin after age 35.
-
SMA Ireland is a national organisation representing people and families affected by Spinal Muscular Atrophy (SMA) across Ireland. Run entirely by volunteers, we work to improve the lives of those living with SMA through advocacy, awareness, and support. Our priorities include securing access to life-changing treatments, advancing newborn screening, improving standards of care, and empowering families with the information and resources they need. We are campaigning to ensure that every person in Ireland with SMA, regardless of their age or background, has the opportunity to access appropriate treatment and to live a full, dignified life. Together with our community, we strive for a future where no one with SMA is left behind.
-
Muscular Dystrophy Ireland is a voluntary, member organisation, founded in 1972. Our mission is to support people with muscular dystrophy and related neuromuscular conditions. We do this by providing information and support to people with neuromuscular conditions and their families; advocating for services and entitlements for members; educating and informing society about neuromuscular conditions; and supporting quality research into neuromuscular conditions.
Contact: treatmentsforall@smaireland.com

Privacy policy Cookie policy Terms & conditions Accessibility statement
©2025